Abstract
Introduction: Although Tyrosine Kinase Inhibitors (TKIs) have changed the natural history of chronic myeloid leukemia (CML), still a significant percentage of patients fail the TKIs available to date. Asciminib is a first-in-class BCR:ABL1 inhibitor specifically targeting the myristoyl pocket of ABL. Since adverse events (AEs) are mostly related to inhibition of non-target kinases by ATP-binding TKIs, asciminib could provide improved tolerability. Asciminib has demonstrated a good safety profile in clinical trials and in some preliminary studies in real clinical practice recently reported by several groups.
Our objective is to perform an analysis focused on the toxicities shown with asciminib, as well as to study the cross-intolerances with other TKIs, which may limit the expected success.
Materials and methods: We retrospectively studied 77 CML patients from 36 centers, who had presented a therapeutic failure to second-generation TKI. All of them received asciminib through a compassionate use program between Oct-2018 and June-2022. Statistical analysis was performed by SPSS using Fisher's exact test.
Results: the mean age at data collection was 66 years. 88% of patients had received ³3 TKIs previously. 34% had previously received ponatinib. Except for one patient who started in accelerated phase, the rest had chronic phase disease. Switching to asciminib was due to intolerance in 64% of patients. 25% had BCR-ABL mutations (4 patients T315I). Asciminib was initiated at a dose of 40mg BID, with the exception of patients with T315I mutation, who were initiated at 200mg BID.
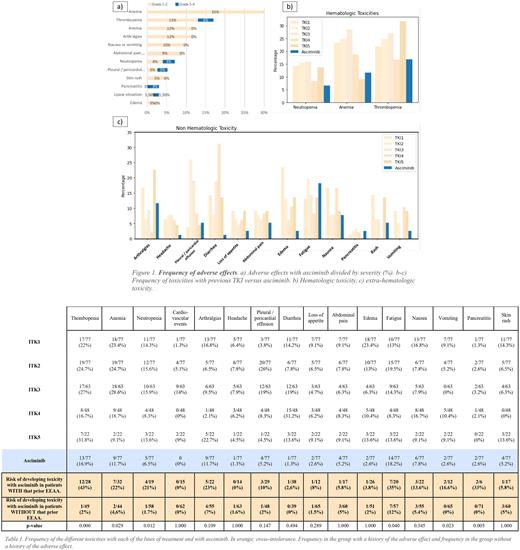
Frequency of AEs is shown in Figure 1. 55% of patients had some AE with asciminib, most of them mild (grades 1-2), with 18% being grade 3-4. Most frequent AE were fatigue (18%), thrombocytopenia (17%), anemia (12%) and arthralgias (12%). None of the patients with previous cardiovascular events presented a new event. There were no cases of de novo occlusive peripheral arterial disease (PAD), although one patient experienced worsening of preexisting PAD.
In 20 patients a dose adjustment was required and in 19 of them a temporary suspension of treatment. The need for dose adjustment was more frequent in the intolerant group than in the group resistant to previous lines (33% vs 14%). Patients who required a dose reduction had an MMR rate at the end of follow-up of 55% (11/20) vs. 63% (34/54) in those who maintained standard doses. 8% (6/77) of patients had to stop treatment definitively due to side effects (pleural effusion, pneumonitis, renal failure, worsening of PAD, two due to pancreatitis).
When comparing AEs of asciminib versus previous classical TKIs, a lower percentage of cytopenias was observed (Fig.1b). For non-hematologic toxicities (Fig.1c), the frequency of AEs was visibly lower for the percentage of headache, pleural/pericardial effusion, diarrhea, and edema. No significant differences were observed for the rate of arthralgias, fatigue, abdominal pain and nausea.
Cross-intolerance with previous TKIs was analyzed for the most frequent AEs observed with asciminib (Table 1). Cross-toxicity risk was statistically significant for thrombopenia, anemia, neutropenia, fatigue, vomiting and pancreatitis. Cross-toxicity does not appear to affect the occurrence of cardiovascular events, edema, abdominal pain, diarrhea or rash. Cross intolerance led to treatment discontinuation in 5 patients (pleural effusion, pneumonitis, worsening of PAD, two cases of pancreatitis).
In terms of efficacy, 73% of patients maintained or achieved a complete cytogenetic response (CCyR) and 60% a major molecular response (MMR). Of the patients who had no prior response, 50% achieved CCyR and 49% achieved MMR. Responses were much better in patients who started asciminib for intolerance versus those who started it for resistance (80% of intolerant patients maintained or achieved MMR vs 26% in the resistant group).
With a median follow-up of 13,74 months a 71% of the patients continued treatment with asciminib. Of the total dropouts 7/22 (9% of total) were due to intolerance, 10/22 due to resistance and 5/22 due to death from any cause.
Conclusion: Asciminib is a molecule with a good safety profile, with a lower rate of AEs compared to classical TKIs. However, this drug, despite its novel mechanism of action presents risk of cross-intolerance with classical TKIs for some toxicities, including hematologic toxicity, fatigue, vomiting and pancreatitis.
Disclosures
Pérez-Lamas:Novartis: Research Funding. Giraldo:Sanofi: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Acindes: Consultancy, Honoraria; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; ZeroLMC Foundation: Honoraria, Membership on an entity's Board of Directors or advisory committees. Lakhwani:Novartis: Honoraria; Janssen: Honoraria, Other: Advisory board. García Gutiérrez:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Incyte: Consultancy, Honoraria, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria, Research Funding, Speakers Bureau; Roche: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal